Lovett Software Services
We are a Zurich-based consulting firm, working with local and international clients. We offer many years’ of experience in large multinational companies and smaller startups.
Our combined engineering and medical insight ensures your products are technically robust and clinically meaningful.
Contact us today to discuss your needs.
Our Services
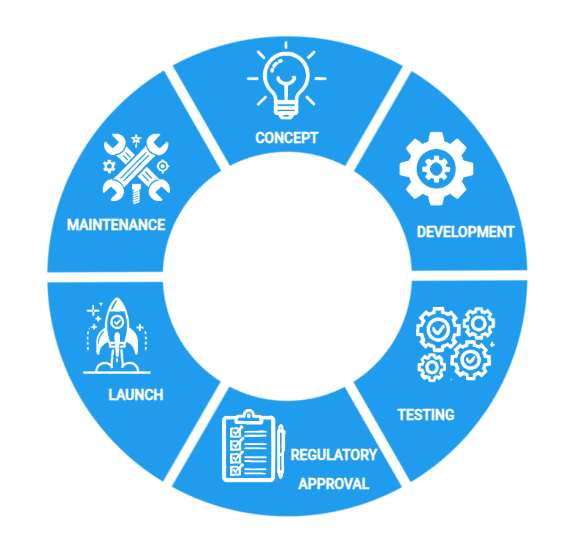
We can support you through every stage of the medical device life-cycle, from concept to product launch and beyond.
Our skills and experience include the following:
Research and Development
Rapid prototyping, patient-centered design, clinical evaluation.
Web and applications development in Typescript, Rust, C# and Python.
Embedded software development in C and Rust.
Cloud-native software architecture, containerization, DevSecOps practicies. HL7 communication standards. ISO 27001 cyber security compliance
Regulatory Affairs
ISO 13485, ISO 14971 and IEC 62304 familiarity
MDR (EU) And FDA 21 CFR Part 820 (USA) compliance
Document control processes and design history files (DHF) in accordance with QMS
Post-market surveillance activities
Sales and Marketing
Providing clinical credibility and authority to establish product credibility and brand trust.
Access to a widespread clinician and expert KOL network.
Exceptional communication and presentation skills to communicate complex medical information to diverse audiences.

